thermodynamics - Confused why delta ∆Q and dQ don't make sense for heat Q - Chemistry Stack Exchange
€ 18.99 · 4.5 (325) · En stock

In my chemistry teacher's notes, some notations concerning the heat $Q$ are marked as inappropriate. $Q$: yes d$Q$: no $\delta Q$: yes $\Delta Q$: no In the second bullet in the screenshot below
Clausius Inequality says the integral of dq/T for a process is less than equal to zero. Also any process needs to have ∆s=dq/T >0. Isn't this contradictory? - Quora
Falkovich Statistical - Physics Notes, PDF, Heat
What is the difference between dq . ∂q . ∆q where q=HEAT? - Quora
Can the Second law of thermodynamics be abandoned?


Essentials of Computational Chemistry

The Laws of Thermodynamics

The Laws of Thermodynamics
Why is 'enthalpy change' (∆H) not always equal to 'heat transfer' (Q)? - Quora
105 questions with answers in STATISTICAL PHYSICS
Can the Second law of thermodynamics be abandoned?
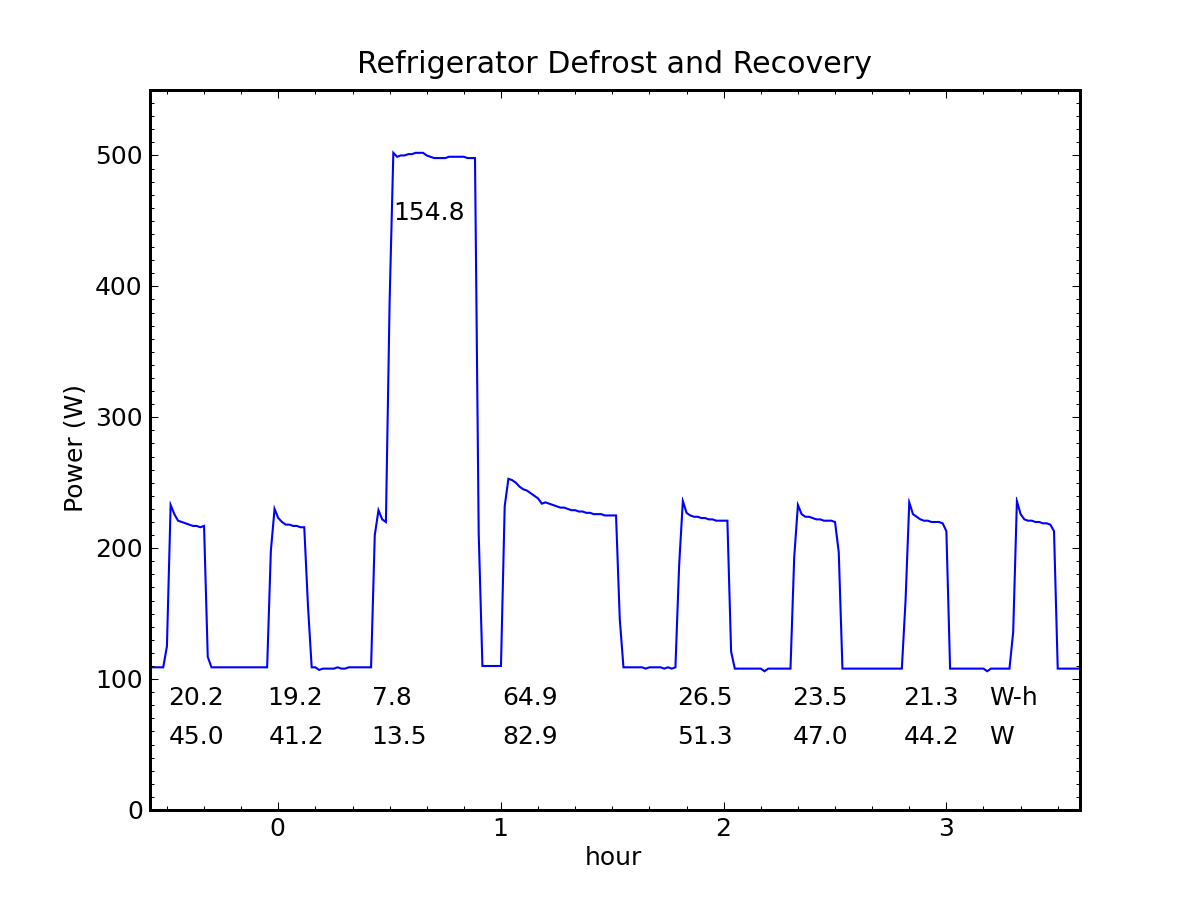
Heat Pumps Work Miracles













